Magnetocaloric properties of lanthanide tetrapyrrole complexes above Curie temperature
Аннотация
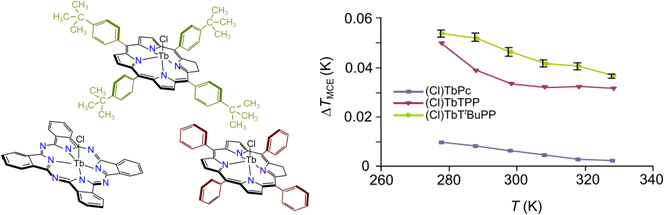
To obtain the fundamental knoledge in the field of magnetocaloric behavior of new molecular materials at room temperature, we have synthesized (5,10,15,20-tetraphenylporphinato)terbium(III), (5,10,15,20-tetra(4-tertbutylphenylporphinato)terbium(III), and (phthalocyaninato)terbium(III) chlorides and have fully characterized their chemical structure using spectral methods (UV-vis, IR, 1H NMR, MALDI TOF). The specific heat capacities of paramagnets synthesized were measured at the temperatures from 224 to 393 K in zero fields by means of DSC method. Magnetocaloric effect, heat, and charge of enthalpy/entropy during the magnetization were observed over the temperature range of 278 - 328 K in magnetic fields from zero to 1 T by the direct microcalorimetric method. Using the analysis of the temperature dependences of the magnetocaloric parameters in lanthanide row, we have established that the control of the magnetic exchange between the spin carrier and the paramagnetic ligand is determined both by the fine tuning of the outer shell of the lanthanide ion and by the substitution in the macrocycle. It was shown at a quantitative level that the terbium ion in the tetrapyrrole complexes carries out the magnetic exchange in 4f75d1 configuration.
Литература
Giménez-Agulló N., de Pipaón C.S., Adriaenssens L., Filibian M., Martínez-Belmonte M., Escudero-Adán E.C., Carretta P., Ballester P., Galán-Mascarós J.R. Chem. Eur. J. 2014, 20, 12817-12825. https://doi.org/10.1002/chem.201402869
Wang H., Wang B.-W., Bian Y. Gao S.J. J. Coord. Chem. Rev. 2016, 306, 195-216. https://doi.org/10.1016/j.ccr.2015.07.004
Magnani N. Int. J. Quant. Chem. 2014, 114, 755-759. https://doi.org/10.1002/qua.24656
Fukuda T., Shigeyoshi N., Yamamura T., Ishikawa N. Inorg. Chem. 2014, 53, 9080-9086. https://doi.org/10.1021/ic501028f
Ishikawa N., Sugita M., Wernsdorfer W. Angew. Chem. Int. Ed. 2005, 44, 2931-2935. https://doi.org/10.1002/anie.200462638
Zhang P., Guo Y.-N., Tang J. Coord. Chem. Rev. 2013, 257, 1728-1763. https://doi.org/10.1016/j.ccr.2013.01.012
Rinehart J.D., Long J.R. Chem. Sci. 2011, 2, 2078-2085. https://doi.org/10.1039/c1sc00513h
Lomova T.N. Axially Сoordinated Metalloporphyrins in Science and Practice. Moscow: Krasand, 2019, 704 p. [Ломова Т.Н. Аксиально координированные металлопорфирины в науке и практике, М.: Красанд, 2019. 704 с.]. https://www.rfbr.ru/rffi/ru/books/o_2087172
Koifman O.I., Ageeva T.A., Beletskaya I.P., Averin A.D., Yakushev A.A., Tomilova L.G., Dubinina T.V., Tsivadze A.Yu., Gorbunova Yu.G., Martynov A.G., Konarev D.V., Khasanov S.S., Lyubovskaya R.N., Lomova T.N., Korolev V.V., Zenkevich E.I., Blaudeck T., von Borczyskowski Ch., Zahn D.R.T., Mironov A.F., Bragina N.A., Ezhov A.V., Zhdanova K.A., Stuzhin P.A., Pakhomov G.L., Rusakova N.V., Semenishyn N.N., Smola S.S., Parfenyuk V.I., Vashurin A.S., Makarov S.V., Dereven’kov I.A., Mamardashvili N.Zh., Kurtikyan T.S., Martirosyan G.G., Burmistrov V.А., Aleksandriiskii V.V., Novikov I.V., Pritmov D.A., Grin M.A., Suvorov N.V., Tsigankov A.A., Fedorov A.Yu., Kuzmina N.S., Nyuchev A.V., Otvagin V.F., Kustov A.V., Belykh D.V., Berezin D.B., Solovieva A.B., Timashev P.S., Milaeva E.R., Gracheva Yu.A., Dodokhova M.A., Safronenko A.V., Shpakovsky D.B., Syrbu S.A., Gubarev Yu.A., Kiselev A.N., Koifman M.O., Lebedeva N.Sh., Yurina E.S. Macroheterocycles 2020, 13, 311-467, https://doi.org/10.6060/mhc200814k
Ishikawa N., Sugita M., Ishikawa T., Koshihara S.-Y., Kaizu Y. J. Am. Chem. Soc. 2003, 125, 8694-8695. https://doi.org/10.1021/ja029629n
Ishikawa N., Sugita M., Okubo T., Tanaka N., Iino T., Kaizu Y. Inorg. Chem. 2003, 42, 2440-2446. https://doi.org/10.1021/ic026295u
Demir S., Meihaus K.R., Long J.R. J. Organomet. Chem. 2018, 857, 164-169. https://doi.org/10.1016/j.jorganchem.2017.10.035
Ishikawa N., Sugita M., Ishikawa T., Koshihara S., Kaizu Y. J. Phys. Chem. B. 2004, 108, 11265-11271. https://doi.org/10.1021/jp0376065
Kizaki K., Ozawa H., Kobayashi T., Matsuoka R., Sakaguchi Y., Fuyuhiro A., Fukuda T., Ishikawa N. Chem. Comm. 2017, 53, 6168-6171. https://doi.org/10.1039/C7CC02960H
Fukuda T., Ozawa H., Sakaguchi Y., Kizaki K., Kobayashi T., Fuyuhiro A., Ishikawa N. Chem. Eur. J. 2017, 23, 16357-16363. https://doi.org/10.1002/chem.201703588
Sakaguchi Y., Kizaki K., Fuyuhiro A., Fukuda T., Ishikawa N. Inorg. Chem. 2018, 57, 15438 - 15444. https://doi.org/10.1021/acs.inorgchem.8b02743
Santria A., Fuyuhiro A., Fukuda T., Ishikawa N. Inorg. Chem. 2017, 56, 10625-10632. https://doi.org/10.1021/acs.inorgchem.7b01546
Santria A., Ishikawa N. Inorg. Chem. 2020, 59, 14326-14336. https://doi.org/10.1021/acs.inorgchem.0c02107
Korolev V.V., Lomova T.N., Korolev D.V., Ramazanova A.G., Mozhzhuhkina E.G., Ovchenkova E.N. In: Environmental Analysis: Applicationals of Nanomatireals (Hussain C.M., Kharisov B., Eds.) Cambridge: Advanced Royal Society of Chemistry 2016, p. 14-47.
Lomova T.N., Korolev V.V., Zakharov A.G. Mat. Sci. Eng. B 2014, 186, 54-63. https://doi.org/10.1016/j.mseb.2014.03.006
Korolev V.V., Lomova T.N., Ramazanova A.G., Korolev D.V., Mozhzhuhkina E.G. J. Organomet. Chem. 2016, 819, 209-215. https://doi.org/10.1016/j.jorganchem.2016.07.002
Korolev V.V., Lomova T.N., Ramazanova A.G., Mozhzhuhkina E.G. Mend. Comm. 2016, 26, 301-303. https://doi.org/10.1016/j.mencom.2016.07.011
Korolev V.V., Lomova T.N., Mozhzhuhkina E.G. Synt. Met. 2016, 220, 502-507. https://doi.org/10.1016/j.synthmet.2016.07.026
Korolev V.V., Lomova T.N., Ramazanova A.G., Balmasova O.V., Mozhzhuhkina E.G. J. Porphyrins Phthalocyanines 2019, 23, 1110-1117. https://doi.org/10.1142/S1088424619501220
Sharples J.W., Collison D. Polyhedron 2013, 66, 15-27. https://doi.org/10.1016/j.poly.2013.08.005
Adler A.D., Longo F.R., Finarelli J.D., Goldmacher J., Assour J., Korsakoff L. J. Org. Chem. 1967, 32, 476. https://doi.org/10.1021/jo01288a053
Bichan N.G., Ovchenkova E.N., Gruzdev M.S., Lomova T.N. J. Struct. Chem. 2018, 59, 711-719. https://doi.org/10.1134/s0022476618030320
Lomova T.N. Russ. J. Inorgan. Chem. 2015, 60, 1123-1128. https://doi.org/10.1134/S0036023615090119
Korolev V.V., Korolev D.V., Ramazanova A.G. J. Therm. Anal. Calorim. 2018, 136, 937-941. https://doi.org/10.1007/s10973-018-7704-y
Korolev V.V., Romanov A.S., Arefyev I.M. Russ. J. Phys. Chem. A. 2006, 80, 464-466. https://doi.org/10.1134/S0036024406030277
Korolev V.V., Klyueva M.E., Arefyev I.M., Ramazanova A.G., Lomova T.N., Zakharov A.G. Macroheterocycles 2008, 1, 68-71. https://doi.org/10.6060/mhc2008.1.68
Pecharsky V.K., Gschneidner Jr. K.A., Pecharsky A.O., Tishin A.M. Phys. Rev. B 2001, 64, 144406. https://doi.org/10.1103/PhysRevB.64.144406
Subbotin N.B., Tomilova L.G., Kostromina N.A., Lukyanets E.A. Zh. Obshch. Khim. 1986, 5, 397-400.
Gouterman M., Hanson L.R., Khalil G.-E., Buchler J.W., Rohbock K., Dophin D. J. Am. Chem. Soc. 1975, 97, 142-149. https://doi.org/10.1021/ja00844a037
Pushkarev V.E., Tomilova L.G, Nemykin V.N. Coord. Chem. Rev. 2016, 319, 110-179. https://doi.org/10.1016/j.ccr.2016.04.005
Korolev V.V., Lomova T.N., Ramazanova A.G., Balmasova O.V., Mozhzhukhina E.G. Synth. Met. 2021, 274, 116696-116702. https://doi.org/10.1016/j.synthmet.2021.116696
Lomova T.N., Klyueva M.E., Koifman O.I. Macroheterocycles 2015, 8, 32-46. https://doi.org/10.6060/mhc140599l
Lomova T.N., Volkova N.I., Berezin B.D. Zh. Neorg. Khim. 1987, 32, 969-974.
Lomova T.N., Berezin B.D., Oparin L.V., Zvezdina V.V. Zh. Neorg. Khim. 1982, 27, 683-688.
Gurek A.G., Basova T., Luneau D., Lebrun C., Kol'tsov E., Hassan A.K., Ahsen V. Inorg. Chem. 2006, 45, 1667-1676. https://doi.org/10.1021/ic051754n
Kittel C. Elementary Statistical Physics. New York: Wiley, 1958.
Pecharsky V.K., Gschneidner Jr K.A., Pecharsky A.O., Tishin A.M. Phys. Rev. B 2001, 64, 144406. https://doi.org/10.1103/PhysRevB.64.144406
Andreenko A.S., Belov K.P., Nikitin S.A., Tishin A.M. Physics-Uspekhi (Advances in Physical Sciences) 1989, 158, 553-579. https://doi.org/10.3367/UFNr.0158.198908a.0553
Lomova T.N., Andrianova L.G., Berezin B.D. Zh. Fiz. Khim. 1987, 61, 2921-2928.
Lomova T.N., Klyueva M.E. Double and Triple Decker Phthalocyanines/Porphyrins. In: Encyclopedia of Nanoscience and Nanotechnology, Vol. 2. (Nalwa H.S., Ed.) Valencia, California, USA: Am. Sci. Publishers, 2004, 565-585.
Lomova T.N., Andrianova L.G. Zh. Neorg. Khim. 1994, 39, 2011-2016.
Remy H. Textbook of Inorganic Chemistry, Vol. 1. (Geest & Portig K.- G., Ed.) Leipzig, Germany: Academic Publishing Society, 1960 [Remy H., Lehrbuch der Anorganischen Chemie, Band I, (Geest & Portig K.- G., Ed.) Leipzig, Deutschland: Akademische Verlagsgesellschaft, 1960].
Korolev V.V., Korolev D.V., Lomova T.N., Mozhzhuhkina E.G., Zakharov A.G. Russ. J. Phys. Chem. A 2012, 86, 504-508. https://doi.org/10.1134/S0036024412030181
Huang S., Dong Z., Mu W., Strom V., Chai G., Varga L.K., Eriksson O., Vitos L. Appl. Phys. Lett. 2021, 119, 141909. https://doi.org/10.1063/5.0065067