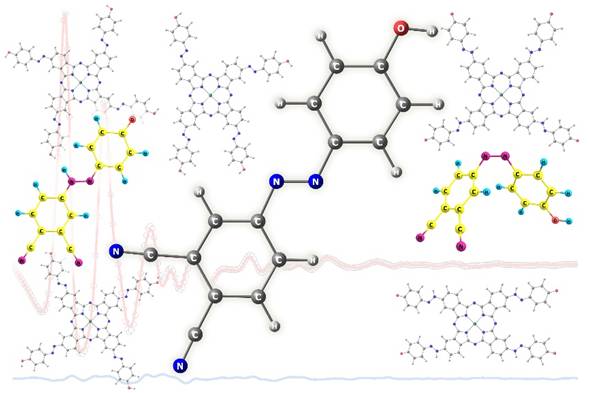
Gas-Phase Structure of 4-(4-Hydroxyphenylazo)phthalonitrile - Precursor for Synthesis of Phthalocyanines with Macrocyclic and Azo Chromophores
Аннотация
Равновесная структура свободных молекул 4-(4-гидроксифенилазо)фталонитрила (p-HPhAPN, C14N4H8O) была впервые исследована с помощью синхронного электронографического-масс-спектрометрического эксперимента, а также с помощью квантово-химических расчетов. Установлено, что p-HPhAPN в паре представлен в виде плоских азо-форм. Цис-транс-изомерия, азо-гидразонная таутомерия и вращения различных фрагментов в p-HPhAPN изучены на теоретических уровнях B3LYP-D3/pcseg-2 и DLPNO-CCSD(T0). Масс-спектр электронного удара p-HPhAPN, являющийся типичным для производных азобензола, был интерпретирован с использованием QCxMS расчетов на уровне теории GFN2-xTB. В связи с возможностями использования p-HPhAPN в получении фталоцианинов, сочетающих в себе макро-циклический и азо- хромофоры, исследовано строение соответствующих фталоцианинатов цинка.
Литература
Ali Y., Hamid A.S., Rashid U. Mini-Reviews Med. Chem. 2018, 18, 1548-1558. https://doi.org/10.2174/1389557518666180524113111
Bafana A., Devi S.S., Chakrabarti T. Environ. Rev. 2011, 19, 350-371. https://doi.org/10.1139/a11-018
Fedele C., Ruoko T.-P., Kuntze K., Virkki M., Priimagi A. Photochem. Photobiol. Sci. 2022, 21, 1719-1734.https://doi.org/10.1007/s43630-022-00262-8
Giles L.W., Faul C.F.J., Tabor R.F. Mater. Adv. 2021, 2, 4152-4164. https://doi.org/10.1039/D1MA00340B
Purkait M.K., Sinha M.K., Mondal P., Singh R. Photoresponsive Membranes. In: Stimuli Responsive Polymeric Membranes, Ch. 4 (Purkait M.K., Sinha M.K., Mondal P., Singh R., Eds.), Academic Press, Elsevier, 2018. pp. 115-144. https://doi.org/10.1016/B978-0-12-813961-5.00004-8
Natansohn A., Rochon P. Chem. Rev. 2002, 102, 4139-4176. https://doi.org/10.1021/cr970155y
Manickasundaram S., Kannan P., Hassan Q.M.A., Palanisamy P.K. J. Mater. Sci. Mater. Electron. 2008, 19, 1045-1053. https://doi.org/10.1007/s10854-007-9450-y
Beharry A.A., Woolley G.A. Chem. Soc. Rev. 2011, 40, 4422-4437. https://doi.org/10.1039/c1cs15023e
Mohr G.J., Müller H., Bussemer B., Stark A., Carofiglio T., Trupp S., Heuermann R., Henkel T., Escudero D., González L. Anal. Bioanal. Chem. 2008, 392, 1411-1418. https://doi.org/10.1007/s00216-008-2428-7
Shikhaliyev N.Q., Kuznetsov M.L., Maharramov A.M., Gurbanov A.V., Ahmadova N.E., Nenajdenko V.G., Mahmudov K.T., Pombeiro A.J.L. CrystEngComm 2019, 21, 5032-5038. https://doi.org/10.1039/C9CE00956F
Naime J., Al Mamun M.S., Aly Saad Aly M., Maniruzzaman M., Badal M.M.R., Karim K.M.R. RSC Adv. 2022, 12, 28034-28042. https://doi.org/10.1039/D2RA04930A
Lee H.Y., Song X., Park H., Baik M.-H., Lee D. J. Am. Chem. Soc. 2010, 132, 12133-12144. https://doi.org/10.1021/ja105121z
Wang X. Trans-Cis Isomerization. In: Azo Polymers. Soft and Biological Matter. Springer Berlin Heidelberg, Berlin, Heidelberg, 2017. pp. 19-56. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-53424-3_2
Tiago M.L., Ismail-Beigi S., Louie S.G. J. Chem. Phys. 2005, 122, 94311. https://doi.org/10.1063/1.1861873
Duarte L., Fausto R., Reva I. Phys. Chem. Chem. Phys. 2014, 16, 16919-16930. https://doi.org/10.1039/C4CP00240G
Fliegl H., Köhn A., Hättig C., Ahlrichs R. J. Am. Chem. Soc. 2003, 125, 9821-9827. https://doi.org/10.1021/ja034433o
Pogonin A.E., Kurochkin I.Y., Malyasova A.S., Ksenofontova K.V., Koifman O.I. Macroheterocycles 2023, 16, 156-167. https://doi.org/10.6060/mhc235113p
Mahimwalla Z., Yager K.G., Mamiya J., Shishido A., Priimagi A., Barrett C.J. Polym. Bull. 2012, 69, 967-1006. https://doi.org/10.1007/s00289-012-0792-0
Shishido A. Polym. J. 2010, 42, 525-533. https://doi.org/10.1038/pj.2010.45
Chang V.Y., Fedele C., Priimagi A., Shishido A., Barrett C.J. Adv. Opt. Mater. 2019, 7, 1900091. https://doi.org/10.1002/adom.201900091
Kishimoto S., Kitahara S., Manabe O., Hiyama H. J. Org. Chem. 1978, 43, 3882-3886. https://doi.org/10.1021/jo00414a020
Ball P., Nicholls C.H. Dyes Pigm. 1982, 3, 5-26. https://doi.org/10.1016/0143-7208(82)80010-7
Özen A.S., Doruker P., Aviyente V. J. Phys. Chem. A 2007, 111, 13506-13514. https://doi.org/10.1021/jp0755645
Steinwand S., Halbritter T., Rastädter D., Ortiz-Sánchez J.M., Burghardt I., Heckel A., Wachtveitl J. Chem. - A Eur. J. 2015, 21, 15720-15731. https://doi.org/10.1002/chem.201501863
Ngororabanga J.M.V., Dembaremba T.O., Mama N., Tshentu Z.R. Spectrochim. Acta, Part A Mol. Biomol. Spectrosc. 2023, 289, 122202. https://doi.org/10.1016/j.saa.2022.122202
Adegoke O.A., Adesuji T.E., Thomas O.E. Spectrochim. Acta, Part A Mol. Biomol. Spectrosc. 2014, 128, 147-152. https://doi.org/10.1016/j.saa.2014.02.118
Baldini L., Balestri D., Marchiò L., Casnati A. Molecules 2023, 28, 4704. https://doi.org/10.3390/molecules28124704
Demaison J., Vogt N. Molecular Structures from Gas-Phase Electron Diffraction. In: Accurate Structure Determination of Free Molecules, Springer International Publishing, Cham, 2020. pp. 167-204. https://doi.org/10.1007/978-3-030-60492-9_7
Girichev G.V., Giricheva N.I., Kudin L.S., Solomonik V.G., Belova N.V., Butman M.F., Vyalkin D.A., Dunaev A.M., Eroshin A.V., Zhabanov Y.A., Krasnov A.V., Kuzmina L.E., Kuzmin I.A., Kurochkin I.Y., Motalov V.B., Navarkin I.S., Pimenov O.A., Pogonin A.E., Sliznev V.V., Smirnov A.N., Tverdova N.V., Shlykov S.A. ChemChemTech [Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol.] 2023, 66(7), 11-30. https://doi.org/10.6060/ivkkt.20236607.6850j
Traetteberg M., Hillmo I., Hagen K. J. Mol. Struct. 1977, 39, 231-239. https://doi.org/10.1016/0022-2860(77)85093-X
Tsuji T., Takashima H., Takeuchi H., Egawa T., Konaka S. J. Phys. Chem. A 2001, 105, 9347-9353. https://doi.org/10.1021/jp004418v
Tikhomirova T.V., Znoiko S.A., Shaposhnikov G.P. Russ. J. Gen. Chem. 2018, 88, 1164-1171. https://doi.org/10.1134/S1070363218060191
Han M., Zhang X., Zhang X., Liao C., Zhu B., Li Q. Polyhedron 2015, 85, 864-873. https://doi.org/10.1016/j.poly.2014.10.026
de la Torre G., Bottari G., Hahn U., Torres T. Functional Phthalocyanines: Synthesis, Nanostructuration, and Electro-Optical Applications. In: Functional Phthalocyanine Molecular Materials (Jiang J., Ed.), Springer Berlin Heidelberg, Berlin, Heidelberg, 2010. pp. 1-44. https://doi.org/10.1007/978-3-642-04752-7_1
Claessens C.G., Hahn U., Torres T. Chem. Rec. 2008, 8, 75-97. https://doi.org/10.1002/tcr.20139
Wöhrle D., Schnurpfeil G., Makarov S.G., Kazarin A., Suvorova O.N. Macroheterocycles 2012, 5, 191-202. https://doi.org/10.6060/mhc2012.120990w
Zhang Y., Lovell J.F. WIREs Nanomedicine and Nanobiotechnology 2017, 9, e1420. https://doi.org/10.1002/wnan.1420
Tverdova N.V., Giricheva N.I., Maizlish V.E., Galanin N.E., Girichev G.V. Int. J. Mol. Sci. 2022, 23, 13922. https://doi.org/10.3390/ijms232213922
Tverdova N.V., Giricheva N.I., Maizlish V.E., Galanin N.E., Girichev G.V. Macroheterocycles 2022, 15, 40-43. https://doi.org/10.6060/mhc214086g
Tyunina V.V., Krasnov A.V., Badelin V.G., Girichev G.V. J. Chem. Thermodyn. 2016, 98, 62-70. https://doi.org/10.1016/j.jct.2016.02.021
Girichev G.V., Utkin A.N., Revichev Y.F. Prib. Tekh. Eksp. 1984, 27, 187-190.
Girichev G.V., Shlykov S.A., Revichev Y.F. Prib. Tekh. Eksp. 1986, 29, 167-169.
Girichev E.G., Zakharov A.V., Girichev G.V., Bazanov M.I. Izv. Vysh. Uchebn. Zaved., Tekst. Prom. 2000, 2, 142-146.
Vishnevskiy Y.V., UNEX [version 1.7], https://unex.vishnevskiy.group (accessed Jan. 10, 2024)
Vishnevskiy Y.V., Zhabanov Y.A. J. Phys. Conf. Ser. 2015, 633, 012076. https://doi.org/10.1088/1742-6596/633/1/012076
Mitzel N.W., Rankin D.W.H. Dalton Trans. 2003, 3650-3662. https://doi.org/10.1039/b307022k
Vishnevskiy Y.V., Abaev M.A., Rykov A.N., Gurskii M.E., Belyakov P.A., Erdyakov S.Y., Bubnov Y.N., Mitzel N.W. Chem. - A Eur. J. 2012, 18, 10585-10594. https://doi.org/10.1002/chem.201200264
Kochikov I.V., Tarasov Y.I., Kuramshina G.M., Spiridonov V.P., Yagola A.G., Strand T.G. J. Mol. Struct. 1998, 445, 243-258. https://doi.org/10.1016/S0022-2860(97)00428-6
Tikhonov D.S., Vishnevskiy Y.V., Rykov A.N., Grikina O.E., Khaikin L.S. J. Mol. Struct. 2017, 1132, 20-27. https://doi.org/10.1016/j.molstruc.2016.05.090
Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., Nakatsuji H., Caricato M., Li X., Hratchian H.P., Izmaylov A.F., Bloino J., Zheng G., Sonnenberg J.L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J.A.,Jr., Peralta J.E., Ogliaro F., Bearpark M., Heyd J.J., Brothers E., Kudin K.N., Staroverov V.N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Rega N., Millam J.M., Klene M., Knox J.E., Cross J.B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R.E., Yazyev O., Austin A.J., Cammi R., Pomelli C., Ochterski J.W., Martin R.L., Morokuma K., Zakrzewski V.G., Voth G.A., Salvador P., Dannenberg J.J., Dapprich S., Daniels A.D., Farkas O., Foresman J.B., Ortiz J.V., Cioslowski J., Fox D.J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009.
Becke A.D. J. Chem. Phys. 1993, 98, 5648-5652. https://doi.org/10.1063/1.464913
Lee C., Yang W., Parr R.G. Phys. Rev. B 1988, 37, 785. https://doi.org/10.1103/PhysRevB.37.785
Grimme S., Antony J., Ehrlich S., Krieg H. J. Chem. Phys. 2010, 132, 154104. https://doi.org/10.1063/1.3382344
Jensen F. J. Chem. Theory Comput. 2014, 10, 1074-1085. https://doi.org/10.1021/ct401026a
Pritchard B.P., Altarawy D., Didier B., Gibson T.D., Windus T.L. J. Chem. Inf. Model. 2019, 59, 4814-4820. https://doi.org/10.1021/acs.jcim.9b00725
Notes about the Jensen basis sets, https://www.basissetexchange.org/family_notes/jensen/ (accessed April 8, 2024).
Pitman S.J., Evans A.K., R Ireland.T., Lempriere F., McKemmish L.K. J. Phys. Chem. A 2023, 127, 10295-10306. https://doi.org/10.1021/acs.jpca.3c05573
Keith T.A. AIMAll (Version 19.10.12), 2017, http://aim.tkgristmill.com/ (accessed May 14, 2021).
Liakos D.G., Guo Y., Neese F. J. Phys. Chem. A 2020, 124, 90-100. https://doi.org/10.1021/acs.jpca.9b05734
Saitow M., Becker U., Riplinger C., Valeev E.F., Neese F. J. Chem. Phys. 2017, 146, 164105. https://doi.org/10.1063/1.4981521
Riplinger C., Neese F. J. Chem. Phys. 2013, 138, 34106. https://doi.org/10.1063/1.4773581
Riplinger C., Sandhoefer B., Hansen A., Neese F. J. Chem. Phys. 2013, 139, 134101. https://doi.org/10.1063/1.4821834
Neese F., Wennmohs F., Becker U., Riplinger C. J. Chem. Phys. 2020, 152, 224108. https://doi.org/10.1063/5.0004608
Minenkov Y., Bistoni G., Riplinger C., Auer A.A., Neese F., Cavallo L. Phys. Chem. Chem. Phys. 2017, 19, 9374-9391. https://doi.org/10.1039/C7CP00836H
Bruno G., de Souza B., Neese F., Bistoni G. Phys. Chem. Chem. Phys. 2022, 24, 14228-14241. https://doi.org/10.1039/D2CP01623K
Dunning T.H. J. Chem. Phys. 1989, 90, 1007-1023. https://doi.org/10.1063/1.456153
Martin J.M.L. Chem. Phys. Lett. 1996, 259, 669-678. https://doi.org/10.1016/0009-2614(96)00898-6
Minenkov Y., Cavallo L., Peterson K.A. J. Comput. Chem. 2023, 44, 687-696. https://doi.org/10.1002/jcc.27033
Weigend F., Köhn A., Hättig C. J. Chem. Phys. 2002, 116, 3175-3183. https://doi.org/10.1063/1.1445115
Grimme S. Angew. Chem. Int. Edit. 2013, 52, 6306-6312. https://doi.org/10.1002/anie.201300158
Koopman J., Grimme S. ACS Omega 2019, 4, 15120-15133. https://doi.org/10.1021/acsomega.9b02011
Bannwarth C., Ehlert S., Grimme S. J. Chem. Theory Comput. 2019, 15, 1652-1671. https://doi.org/10.1021/acs.jctc.8b01176
Giricheva N.I., Lebedev I.S., Fedorov M.S., Bubnova K.E., Girichev G.V. J. Struct. Chem. 2021, 62, 1976-1987. https://doi.org/10.1134/S0022476621120179
Islyaikin M.K., V Ferro.R., García de la Vega J.M. J. Chem. Soc., Perkin Trans. 2 2002, 17, 2104-2109. https://doi.org/10.1039/B207034K
In: NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology.
Moran M.J., Martina K., Baricco F., Tagliapietra S., Manzoli M., Cravotto G. Adv. Synth. Catal. 2020, 362, 2689-2700. https://doi.org/10.1002/adsc.202000127
Zhang C., N. Jiao, Angew. Chem. Int. Edit. 2010, 49, 6174-6177. https://doi.org/10.1002/anie.201001651
Bowie J.H., Lewis G.E., Cooks R.G. J. Chem. Soc. B 1967, 621-628. https://doi.org/10.1039/j29670000621
Schreckenbach S.A., Anderson J.S.M., Koopman J., Grimme S., Simpson M.J., Jobst K.J. J. Am. Soc. Mass Spectrom. 2021, 32, 1508-1518. https://doi.org/10.1021/jasms.1c00078
Bowie J.H., Lawesson S.O., Madsen J.Ø., Nolde C., Schroll G., Williams D.H. J. Chem. Soc. B 1966, 946-951. https://doi.org/10.1039/j29660000946
Vogt N., Savelev D., Giricheva N.I., Girichev G.V. Phys. Chem. Chem. Phys. 2020, 22, 27539-27546. https://doi.org/10.1039/D0CP04423G