Synthesis and Structure Elucidation of β,β,β-Trinitro-meso-tetraphenylporphyrin Derivatives
Аннотация
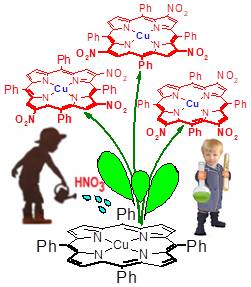
The preparation, chromatographic isolation, and structure elucidation of β,β,β-substituted isomers of trinitro-5,10,15,20-tetraphenylporphyrin complexes are described. meso-Tetraphenylporphyrin (m-TPP) chelates (CuII and ZnII) in the reaction with nitric acid (yellow fuming HNO3, d = 1.52) in CHCl3 resulted in the formation of a mixture of nitroderivatives with combined yields of ca 50%. This nitration (under optimized conditions: 1–2 min, r.t.) can be carried out selectively to give mainly β,β,β-trinitro-compounds in the yield of up to 30-40%. From the above mixtures of seven possible regioisomers to form, two or three of them were usually isolated for which the structures were assigned on the basis of 1H NMR spectra including HSQC and HMBC measurements. These types of products are attractive starting materials for synthesis of potential anticancer PDT agents of unique structure that are practically not available by any other alternative method
Литература
Handbook of Porphyrin Science (Kadish K.M., Smith K.M., Guilard R., Eds.), New Jersey-London-Singapore-Beijing-Shanghai-Hong Kong-Taipei-Chennai: World Scientific Publishing Co., 2010-2012, Vols. 1-25.
Moser J.G. Photodynamic Tumor Therapy: 2nd and 3rd Generation Photosensitizers, Amsterdam: Harwood Academic Publishers, 1998.
Hsi R.A., Rosenthal D.I., Glatstein E. Drugs 1999, 57, 725-734. https://doi.org/10.2165/00003495-199957050-00005
Correia J.H., Rodrigues J.A., Pimenta S., Dong T., Yang Z. Pharmaceutics 2021, 13, 1332. https://doi.org/10.3390/pharmaceutics13091332
Monteiro C.J.P., Pina J., Pereira M.M., Arnaut L.G. Photochem. Photobiol. Sci. 2012, 11, 1233-1238. https://doi.org/10.1039/c2pp25021g
Wei L., Padmaja K., Youngblood W.J., Lysenko A.B., Lindsey J.S., Bocian D.F. J. Org. Chem. 2004, 69, 1461-1469; and refs. cited therein. https://doi.org/10.1021/jo0349476
Ruzié C., Gueyrard D., Boitrel B. Tetrahedron Lett. 2004, 45, 1713-1716; and refs. cited therein. https://doi.org/10.1016/j.tetlet.2003.12.100
Imahori H., Hagiwara K., Aoki M., Akiyama T., Taniguchi S., Okada T., Shirakawa M., Sakata Y. J. Am. Chem. Soc. 1996, 118, 11771-11782. https://doi.org/10.1021/ja9628415
Zheng G., Dougherty T.J., Pandey R.K. Chem. Commun. 1999, 2469-2470. https://doi.org/10.1039/a906889i
Ostrowski S., Mikus A. Mol. Divers. 2003, 6, 315-321. https://doi.org/10.1023/B:MODI.0000006864.22321.2e
Malinovskii V.L., Vodzinskii S.V., Zhilina Z.I., Andronati S.A., Mazepa A.V. Zh. Org. Khim. 1996, 32, 119-123.
Ostrowski S., Raczko A.M. Helv. Chim. Acta 2005, 88, 974-978. https://doi.org/10.1002/hlca.200590091
Ostrowski S., Grzyb S. Tetrahedron Lett. 2012, 53, 6355-6357. https://doi.org/10.1016/j.tetlet.2012.09.024
Mąkosza M. Chem. Soc. Rev. 2010, 39, 2855-2868. https://doi.org/10.1039/b822559c
Ostrysz S., Mikus A., Ostrowski S. Macroheterocycles 2017, 10, 323-327. https://doi.org/10.6060/mhc170405o
Dahal S., Krishnan V., Nethaji M. Proc. Indian Acad. Sci. Chem. Sci. 1998, 110, 37-52. https://doi.org/10.1007/BF02871908
Rosa M., Ostrowski S. ChemistrySelect 2022, 7: e202200290. https://doi.org/10.1002/slct.202200290
Evans B., Smith K.M., Cavaleiro J.A.S. J. Chem. Soc., Perkin Trans. 1 1978, 768-773. https://doi.org/10.1039/p19780000768
Baldwin J.E., Crossley M.J., DeBernardis J. Tetrahedron 1982, 38, 685-692. https://doi.org/10.1016/0040-4020(82)80211-1
Crossley M.J., Harding M.M., Sternhell S. J. Am. Chem. Soc. 1986, 108, 3608-3613. https://doi.org/10.1021/ja00273a010
Karelson M., Pihlaja K., Tamm T., Uri A., Zerner M.C. J. Photochem. Photobiol A: Chem. 1995, 85, 119-126. https://doi.org/10.1016/1010-6030(94)03896-3
Blom M., Norrehed S., Andersson C.-H., Huang H., Light M.E., Bergquist J., Grennberg H., Gogoll A. Molecules 2016, 21, 16. https://doi.org/10.3390/molecules21010016
Ostrowski S. Polish J. Chem. 2005, 79, 1169-1172.
Mikus A., Rosa M., Ostrowski S. Molecules 2019, 24, 838. https://doi.org/10.3390/molecules24050838
Mikus A., Ostrowski S. Struct. Chem. 2022, 33, 1251-1255. https://doi.org/10.1007/s11224-022-01951-x
Mikus A., Ostrowski S. Struct. Chem. 2022, 33, 2261. https://doi.org/10.1007/s11224-022-01971-7
Kolodina E.A., Syrbu S.A., Semeikin A.S., Koifman O.I. Russ. J. Org. Chem. 2010, 46, 138-143. https://doi.org/10.1134/S107042801001015X
Mikus A., Łopuszyńska B. Chem. Asian J. 2021, 16, 261-276. https://doi.org/10.1002/asia.202000985
Ostrowski S., Szerszeń D., Ryszczuk M. Synthesis 2005, 37, 819-823. https://doi.org/10.1055/s-2005-861853
Fuhrhop J.-H., Smith K.M. In Porphyrins and Metalloporphyrins, Smith K.M. Ed., Elsevier: Amsterdam, 1975, pp. 795-811.