Molecular Details of the Interaction of Sorafenib with 2-Hydroxypropyl-β-cyclodextrin
Аннотация
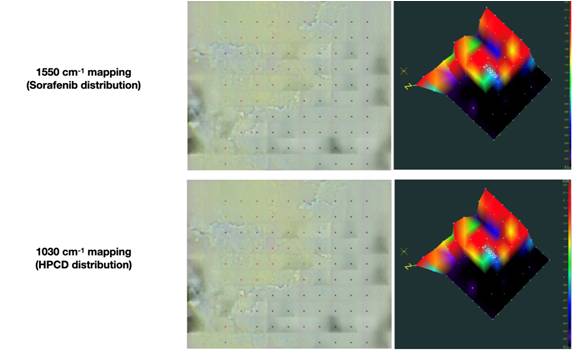
Present study addresses the molecular aspects of the interaction between the anticancer drug sorafenib and 2-hydroxy-propyl-β-cyclodextrin. Formation of the complex was confirmed by means of powder X-ray diffraction. Infrared microscopy confirmed uniform distribution of sorafenib and 2-hydroxypropyl-β-cyclodextrin. The detailed mechanism of complex formation in an aqueous media was examined using ATR-FTIR spectroscopy. The inclusion of the pyridine ring of sorafenib in the tori of cyclodextrin has been demonstrated, as well as the interaction of the active molecule with the sugar backbone of the carrier. Obtained results provided new details in the mechanisms of complex formation between anticancer drugs and cyclodextrin’s torus.
Литература
Iyer R., Fetterly G., Lugade A., Thanavala Y. Expert Opin. Pharmacother. 2010, 11, 1943-1955. https://doi.org/10.1517/14656566.2010.496453
Jiang S., Qin Y., Wu S., Xu S., et al. J. Chem. Eng. Data 2017, 62, 259-267. https://doi.org/10.1021/acs.jced.6b00630
Guo Y., Zhong T., Duan XC. Zhang S., et al. Drug Delivery 2017, 24, 270-277. https://doi.org/10.1080/10717544.2016.1245371
Su Y., Wang K., Li Y., Song W., et al. Nanomedicine 2018, 13, 0046. https://doi.org/10.2217/nnm-2018-0046
Davis M.E., Brewster M.E. Nat. Rev. Drug Discov. 2004, 3, 1023-1035. https://doi.org/10.1038/nrd1576
Bondi M.L., Scala A., Sortino G., Amore E., et al. Biomacromolecules. 2015, 16, 3784-3791.
https://doi.org/10.1021/acs.biomac.5b01082
Aman A., Ali S., Mahalapbutr P., Krusong K., et al. RSC Advances. 2023, 13, 27244 -27254. https://doi.org/10.1039/D3RA03867J
Shukla S., Goyal M., Kanabar D., Ayehuniein S., et al. J. Mol. Liq. 2024, 401, 124701. https://doi.org/10.1016/j.molliq.2024.124701
Bui V.C., Pham T.L., Nguyen T.L., Tran T.K.C., Le T.M.H., Vu X.M., Deygen I.M., Nguyen C.A., Mai T.T., Shuib R.K. Pure Appl. Chem. 2024, 96, 1091-1099. https://doi.org/10.1515/pac-2024-0024
Deygen I.M., Seidl C., Koelmel D.K., Bednarek C., et al. Langmuir 2016, 32, 10861-10869. https://doi.org/10.1021/acs.langmuir.6b01023
Skuredina A.A., Tychinina A.S., Le-Deygen I.M., Golyshev S.A., Belogurova N.G., Kudryashova E.V. React. Funct. Polym. 2021, 159, 104811. https://doi.org/10.1016/j.reactfunctpolym.2021.104811
Ebadi M., Bullo S., Buskara K., Hussein M.Z., Fakurazi S., Pastorin G. Sci. Rep. 2020, 10, 21521. https://doi.org/10.1038/s41598-020-76504-5
Truong D.H., Tran T.H., Ramasamy T., Choi J.Y., Choi H.G., Yong C.H., Kim J.O. Powder Technol. 2015, 283, 260-265. https://doi.org/10.1016/j.powtec.2015.04.044
Ruman U., Buskaran K., Pastorin G., Masarudin M.J., Fakurazi S., Hussein M.Z. Nanomaterials 2021, 11, 497. https://doi.org/10.3390/nano11020497
Periasamy R., Nayaki S.K., Sivakumar K., Ramasamy G. J. Mol. Liq. 2020, 316, 113843. https://doi.org/10.1016/j.molliq.2020.113843
Deygen I.M., Skurending A.A., Kudryashova E.V. Anal Bioanal. Chem. 2017, 409, 6451-6462. https://doi.org/10.1007/s00216-017-0590-5
Grdadolnik J., Marechal Y. J. Mol. Struct. 2002, 615, 177-189. https://doi.org/10.1016/S0022-2860(02)00214-4
Deygen I.M., Safronova A.S., Kolmogorov I.M., Skuredina A.A., Kudryashova E.V. Russ J. Bioorg. Chem. 2022, 48, 710-719. https://doi.org/10.1134/S1068162022040148