Antimicrobial Photodynamic Therapy Activity Properties of 2,6-Brominated and -Iodinated BODIPY Core Dyes and their π-Extended 3,5-Distyryl Analogues
Аннотация
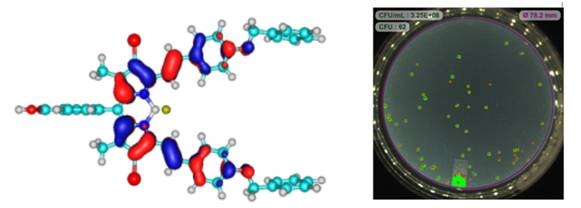
The photodynamic antimicrobial chemotherapy (PACT) activity properties of 2,6-dibrominated and -diiodinated meso-methyl phenyl ester BODIPY dyes π-extended with 3,5-p-dibenzyloxystyryl groups at the 3,5-positions were investigated against Gram-(+) Staphylococcus aureus and Gram-(−) Escherichia coli bacteria and Candida albicans fungus using LEDs with output maxima at 660 and 530 nm. The core dyes were found to have significantly higher PACT activities than the π-extended dyes in the context of the Gram-(+) and -(−) bacterial strains, while low PACT activity was also observed against Candida albicans with the 2,6-dibrominated core dye. The results are consistent with the trends reported in a previous study with BODIPY core and 3,5-divinylene dyes and provide further evidence that research related to the PACT activity properties of BODIPYs should focus primarily on halogenated core dyes. The use of a methyl-β-cyclodextrin inclusion complex was found to significantly enhance the aqueous solubility and PACT activity of the 2,6-diiodinated with 3,5-p-dibenzyloxystyryl groups at the 3,5-positions.
Литература
Mamone L., Ferreyra D.D., Gándara L., Di Venosa G., Vallecorsa P., Sáenz D. J. Photochem. Photobiol. B. 2016, 161, 222-229. https://doi.org/10.1016/j.jphotobiol.2016.05.026
Xu Z., Gao Y., Meng S., Yang B., Pang L., Wang C., Liu T. Front. Microbiol. 2016, 7, 242. https://doi.org/10.3389/fmicb.2016.00242
Liu Y., Qin R., Zaat S.A.J., Breukink E., Heger M. J. Clin. Transl. Res. 2015, 1, 140-167. https://doi.org/10.18053/jctres.201503.002
Cieplik, F., Deng, D., Crielaard, W., Buchalla, W., Hellwig, E., Al-Ahmad, A.,Maisch, T. Crit. Rev. Microbiol. 2018, 44, 571-589. https://doi.org/10.1080/1040841X.2018.1467876
Youf R., Müller M., Balasini A., Thétiot F., Müller M., Hascoët A., Jonas U., Schönherr H., Lemercier G., Montier T., Le Gall T. Pharmaceutics 2021, 13, 1995. https://doi.org/10.3390/pharmaceutics
Treibs A., Kreuzer F.H. Justus Liebigs Ann. Chem. 1968, 718, 208-223. https://doi.org/10.1002/jlac.19687180119
Arbeloa T.L., Arbeloa F.L., Arbeloa I.L., Garcia-Moreno I., Costela A., Sastre R., Amat-Guerri F. Chem. Phys. Lett. 1999, 299, 315-321. https://doi.org/10.1016/S0009-2614(98)01281-0
Ngoy B.P., May A.K., Mack J., Nyokong, T. J. Mol. Struct. 2019, 1175, 745-753. https://doi.org/10.1016/j.molstruc.2018.08.012
Harris J., May A.K., Ngoy B.P., Mack J., Nyokong, T. J. Porphyrins Phthalocyanines 2019, 23, 63-75. https://doi.org/10.1142/S1088424619500019
Trieflinger C., Rurack K., Daub J. Angew. Chem. Int. Ed. 2005, 44, 2288-2291. https://doi.org/10.1002/anie.200462377
Gabe Y., Ueno T., Urano Y., Kojima H., Nagano T. Anal. Biochem. 2006, 386, 621-626. https://doi.org/10.1007/s00216-006-0587-y
Bomanda B.T., Waudo W., Ngoy B.P., Muya J.T., Mpiana P.T., Mbala M., Openda I., Mack J., Nyokong T. Macroheterocycles 2018, 11, 429-437. https://doi.org/10.6060/mhc180898n
May A.K., Ngoy B.P., Mack J., Nyokong T. J. Porphyrins Phthalocyanines 2024, 28, 88-96. https://doi.org/10.1142/S1088424623501316
Sen P., Sindelo A., Nnaji N., Mack J., Nyokong T. Photochem. Photobiol. 2023, 99, 947-956. https://doi.org/10.1111/php.13698
Ngoy B.P., Hlatshwayo Z., Nwaji N., Fomo G., Mack J., Nyokong T. J. Porphyrins Phthalocyanines 2018, 22, 413-422. https://doi.org/10.1142/S1088424617500857
Saokham P., Muankaew C., Jansook P., Loftsson T. Molecules 2018, 23, 1161. https://doi.org/10.3390/molecules23051161
Gandra N., Frank A.T., Le Gendre O., Sawwan N., Aebisher D., Liebman J.F., Houk K.N., Greer A., Gao R. Tetrahedron 2006, 62, 10771−10776. https://doi.org/10.1016/j.tet.2006.08.095
Ogunsipe A., Maree D., Nyokong T. J. Mol. Struct. 2003, 650, 131−140. https://doi.org/10.1016/S0022-2860(03)00155-8
Fischer M., Georges J. Chem. Phys. Lett. 1996, 260, 115−118. https://doi.org/10.1016/0009-2614(96)00838-X
Jiao L., Pang W., Zhou J., Wei Y., Mu X., Bai G., Hao E. J. Org. Chem. 2011, 76, 9988−9996. https://doi.org/10.1021/jo201754m
Ngoy B.P., Molupe N., Harris J., Fomo G., Mack J., Nyokong T. J. Porphyrins Phthalocyanines 2017, 21, 431−438. https://doi.org/10.1142/S1088424617500420
Zhu S., Zhang J., Vegesna G., Tiwari A., Luo F.-T., Zeller M., Luck R., Li H., Green S., Liu H. RSC Adv. 2012, 2, 404−407. https://doi.org/10.1039/C1RA00678A
Miclea L.-M., Vlaia L., Vlaia V., Hădărugă D.I., Mircioiu C. Farmacia 2010, 58, 583−593.
Hedges A.R. Chem. Rev. 1998, 98, 2035−2044. https://doi.org/10.1021/cr970014w
Molupe N., Babu B., Prinsloo E., Kaassis A., Edkins K., Mack J., Nyokong T. J. Porphyrins Phthalocyanines 2019, 23, 1486−1494. https://doi.org/10.1142/S1088424619501633
Sindelo A., Osifeko O.L., Nyokong T. Inorg. Chim. Acta 2018, 476, 68−76. https://doi.org/10.1016/j.ica.2018.02.020
Chavez-Esquivel G., Cervantes-Cuevas H., Ybieta-Olvera L.F., Briones M.C., Acosta D., Cabello, J. Mater. Sci. Eng. C, 2021, 123, 111934. https://doi.org/10.1016/j.msec.2021.111934
Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., Nakatsuji H., Caricato M., Li X., Hratchian H.P., Izmaylov A.F., Bloino, J., Zheng, G., Sonnenberg J.L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J.A. Jr, Peralta J.E., Ogliaro F., Bearpark M., Heyd J.J., Brothers E., Kudin K.N., Staroverov V.N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Rega N., Millam J.M., Klene M., Knox J.E., Cross J.B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R.E., Yazyev O., Austin A.J., Cammi R., Pomelli C., Ochterski J.W., Martin R.L., Morokuma K., Zakrzewski V.G., Voth G.A., Salvador P., Dannenberg J.J., Dapprich S., Daniels A.D., Farkas Ö., Foresman J.B., Ortiz J.V., Cioslowski J., Fox D.J. Gaussian 09, Revision E.01, Gaussian Inc., Wallingford CT, 2009.
Grimme S., Antony J., Ehrlich S., Krieg H. J. Chem. Phys. 2010, 132, 154104. https://doi.org/10.1063/1.3382344
Lu H., Mack J., Yang Y., Shen Z. Chem. Soc. Rev. 2014, 43, 4778−4823. https://doi.org/10.1039/C4CS00030G
Ngoy B.P., May A.K., Mack J., Nyokong T. Front. Chem. 2019, 7, 740. https://doi.org/10.3389/fchem.2019.00740
Wardlaw J.L., Sullivan T.J., Lux C.N., Austin F.W. Vet. J. 2012, 192, 374−377https://doi.org/10.1016/j.tvjl.2011.09.007
Ryskova L., Buchta V., Slezak R. Central Eur. J. Biology 2010, 5, 400-406. https://doi.org/10.2478/s11535-010-0032-2
Ghorbani J., Rahban D., Aghamiri S., Teymouri A., Bahador A. Laser Ther. 2018, 27, 293-302. https://doi.org/10.5978/islsm.27_18-RA-01
Sperandio F.F., Huang Y.Y., Hamblin M.R. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108-120. https://doi.org/10.2174/1574891X113089990012
Zgurskaya H.I., López C.A., Gnanakaran S. ACS Infect. Dis. 2016, 1, 512-522. https://doi.org/10.1021/acsinfecdis.5b00097
Cieplik F., Deng D., Crielaard W., Buchalla W., Hellwig E., Al-Ahmad A., Maisch T. Crit. Rev. Microbiol. 2018, 44, 571-589. https://doi.org/10.1080/1040841X.2018.1467876
Poulain D. Crit. Rev. Microbiol. 2015, 41, 208-17. https://doi.org/10.3109/1040841X.2013.813904